《Balanced Solvation/De-solvation of Electrolyte Facilitates Li-ion Intercalation for Fast Charging and Low-temperature Li-ion Batteries》
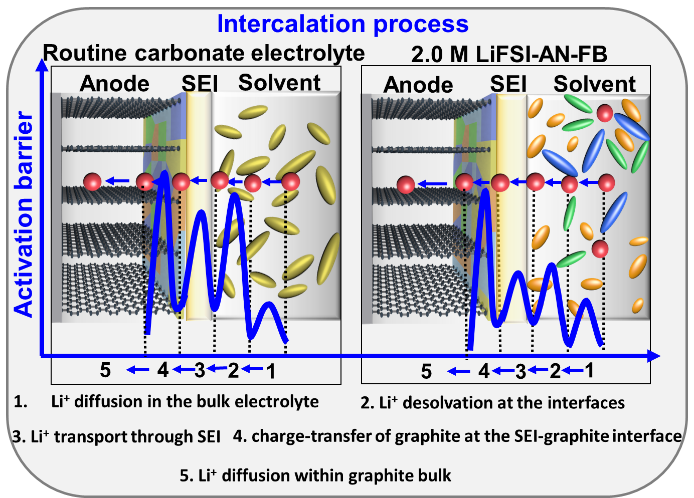
Long charging times and poor low-temperature performance are two major challenges that hamper the widespread use of lithium-ion batteries in electrical devices. The electrolyte plays an important role in determining the charging time and operating temperature of batteries. Herein we demonstrate a weakly-solvating electrolyte consisting of 2.0 M lithium bis(fluorosulfonyl)imide in acetonitrile with fluorobenzene as the cosolvent. This combination is superior in terms of balancing the solvation/de-solvation of an electrolyte which simultaneously yields enhanced diffusion of Li+ in the bulk electrolyte and improved kinetics of Li+ de-solvation. In addition, we achieve a rapid interfacial diffusion of Li+ at the inorganic–polymeric solid electrolyte interphase, derived from fluorobenzene. Graphite half cells show a high specific capacity of 302.7 mA h g−1 at 8 C, long-term cycle life (91% retention after 1000 cycles at 5 C), and remarkable low temperature performance. Moreover, the NCM811 | graphite pouch cells also exhibit outstanding performance for fast-charging (201mA h g−1 at 0.5 C and 167 mA h g−1 at 5 C) as well as outstanding cycling stability (80% retention after 500 cycles at 5 C). In summary, we provide design principles and experimental demonstration of next-generation electrolytes capable of fast charging and low-temperature operation.
https://www.sciencedirect.com/science/article/pii/S2211285522003445
