《Non-polar Co-solvent Driving LUMO Energy Evolution of Methyl Acetate Electrolyte to Afford Lithium-ion Batteries Operating at −60°C》
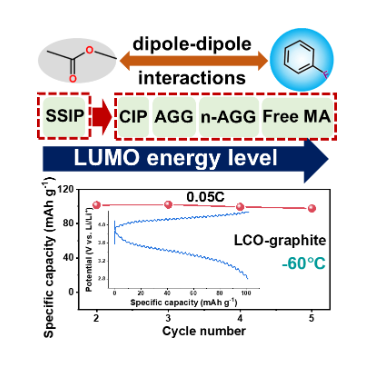
The operation of lithium-ion batteries (LIBs) at low temperatures (<−20°C) is hampered by the low conductivity and high viscosity of conventional carbonate electrolytes. Methyl acetate (MA) has proven to be a competitive low-temperature electrolyte solvent with low viscosity and low freezing point, but its interfacial stability is poor and has been difficult to figure out until now. Here, we reveal that the reduction stability of MA-based electrolytes is fundamentally governed by the anion-dominated solvation structure. Based on this framework, fluorobenzene was employed in the electrolyte to facilitate the entry of anions into the solvated shell through the dipole-dipole interaction and to produce free MA, thereby increasing the LUMO energy level of MA. The designed electrolyte enables lithium cobalt oxide (LCO)/graphite batteries to exhibit excellent cycling performance at -20°C (90% after 1000 cycles at 1°C) and 91% room temperature capacity at 0.05C at ultra-low temperature of -60°C. Thanks to the abundance of free MA, this electrolyte has a high conductivity (2.61 mS cm-1) at -60 °C, allowing LCO/graphite cells to be charged at -60 °C. This work opens up practical possibilities for solvents with poor reduction stability and provides new approaches for designing advanced electrolytes for cryogenic applications.
https://onlinelibrary.wiley.com/doi/10.1002/adfm.202301028
