《Nonflammable Cosolvent Enables Methyl Acetate-based Electrolyte for 4.6 V-class Lithium-ion Batteries Operating at −60°C》
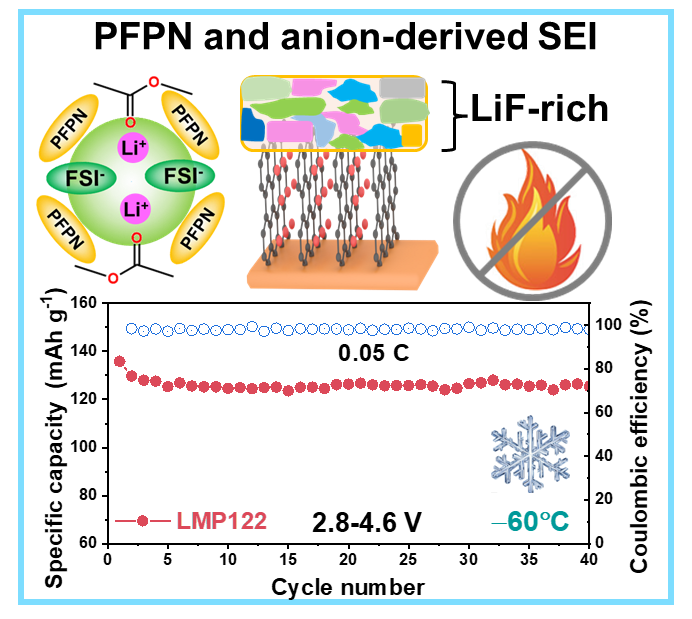
Abstract: The operational demands of electronic devices in extreme environments present a significant challenge for lithium-ion batteries (LIBs) to function effectively at low temperatures. To address this challenge, methyl acetate (MA) has been considered as a potential electrolyte solvent. However, MA-based electrolytes show poor compatibility with graphite anodes and high flammability, which affects battery cycle life and brings safety risks. Herein, an advanced non-flammable MA-based diluted highly concentrated electrolyte (DHCE) is designed incorporating ethoxy (pentafluoro) cyclotriphosphazene (PFPN) as a multifunctional diluent. The MA-based DHCE forms an anion-dominated solvation structure with improved thermodynamic stability. Moreover, PFPN synergizes with anions to form a protective LiF-rich solid electrolyte interphase (SEI), which effectively prevents continuous solvent decomposition. More importantly, PFPN can quench the free radical reaction and stop the spread of flames. This electrolyte enables LiNi0.65Co0.15Mn0.2O2 (NCM65)/graphite full cell to maintain 94.6% capacity retention after 300 cycles at 1 C and −20 °C. Moreover, it allows NCM65/graphite full cell to operate at a high voltage of 4.6 V and −60 °C. This work demonstrates the importance of co-solvents for electrolyte design and provides new routes for the development of LIBs operating in harsh conditions.
https://www.sciencedirect.com/science/article/pii/S1385894723059119?via%3Dihub
