《Anion-Regulated Weakly Solvating Electrolytes for High-Voltage Lithium Metal Batteries》
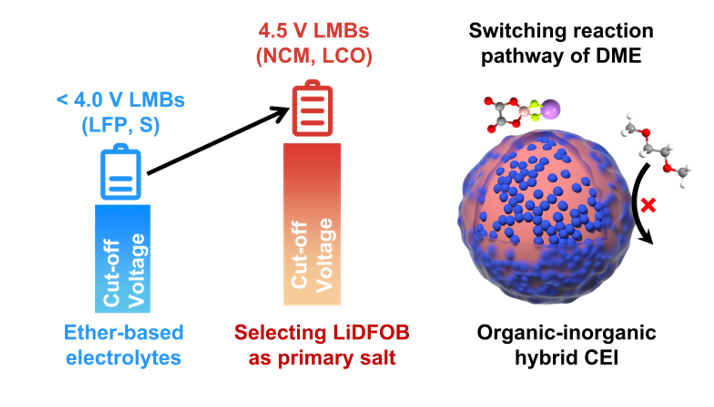
The development of high-voltage lithium-metal batteries (LMBs) is inseparable from the design of new electrolytes. Compared with carbonate electrolytes, ether electrolytes have better stability for lithium metal anodes (LMAs), but their oxidation stability is poor. The traditional view has always been that ether electrolytes with medium concentrations (~1M) cannot directly match high voltages in cathode materials such as NCM and LCO. Although researchers in recent years have improved the high-voltage stability of ether electrolytes through strategies like developing high-concentration electrolytes (HCEs), localized high-concentration electrolytes (LHCEs), and weakly solvating electrolytes (WSEs), these methods usually increase the cost of the electrolyte and sacrifice the high conductivity advantage of ether electrolyte. Therefore, there is an urgent need to explore new methods that can achieve high-voltage stability in ether electrolytes. In this work, we found that when using LiDFOB as the main salt, the ether electrolyte can break through the traditional voltage limit and improve oxidation stability from 4.0 V to 4.5 V. This is because LiDFOB can change the decomposition path of the ether electrolyte and form a stable interface protective layer before the solvent is decomposed. Thanks to this advantage, LiDFOB-based ether electrolyte can enable Li-LiCoO2 batteries (cutoff voltage 4.5 V) to cycle stably 1000 times at 2 C with a capacity retention rate of 86%.
Links::https://pubs.acs.org/doi/10.1021/acs.nanolett.3c02013
