《Pairing non-solvating cosolvent with weakly solvating solvents for facile desolvation to enable EC-free and high-rate electrolyte for lithium ion batteries》
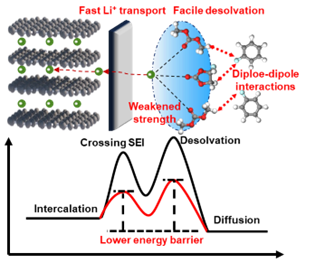
The advancement of lithium-ion batteries (LIBs) calls for superior electrolyte design to satisfy the booming demands of energy storage markets. Despite being typically indispensable in modern electrolytes, ethylene carbonate (EC) exhibits strong affinity with Li+ and derives organic-rich SEI, leading to sluggish interfacial dynamics and unsatisfied rate capability. Here, we construct a kinetically favorable electrolyte based on linear carbonates (DMC) and fluoroethylene carbonate (FEC) with functional non-solvating cosolvent, fluorobenzene (FB). Specifically, DMC and FEC, as weak binding solvents, show facile interfacial kinetics. FB enables a loosen coordination chemistry through dipole-dipole interaction, further lubricating Li+ desolvation and transport through SEI at interphase, which accelerates electrochemical kinetics and enables improved rate performance (257 mA h g−1 is achieved for graphite at 6C). Moreover, the optimized electrolyte shows decent graphite compatibility, long-term stability (75% capacity retention after 350 cycles for NCM622/graphite pouch cells at 1C) and wide-temperature adaptability (−40~160 °C). This work reveals that dipole-dipole interaction between non-solvating cosolvents and solvents can effectively lower desolvation energy barrier and ultimately enable good rate capability, which provides a new avenue for designing EC-free electrolyte in high-rate LIBs.