《Doping in Solvation Structure: Enabling Fluorinated Carbonate Electrolyte for High-Voltage and High-Safety Lithium-Ion Batteries》
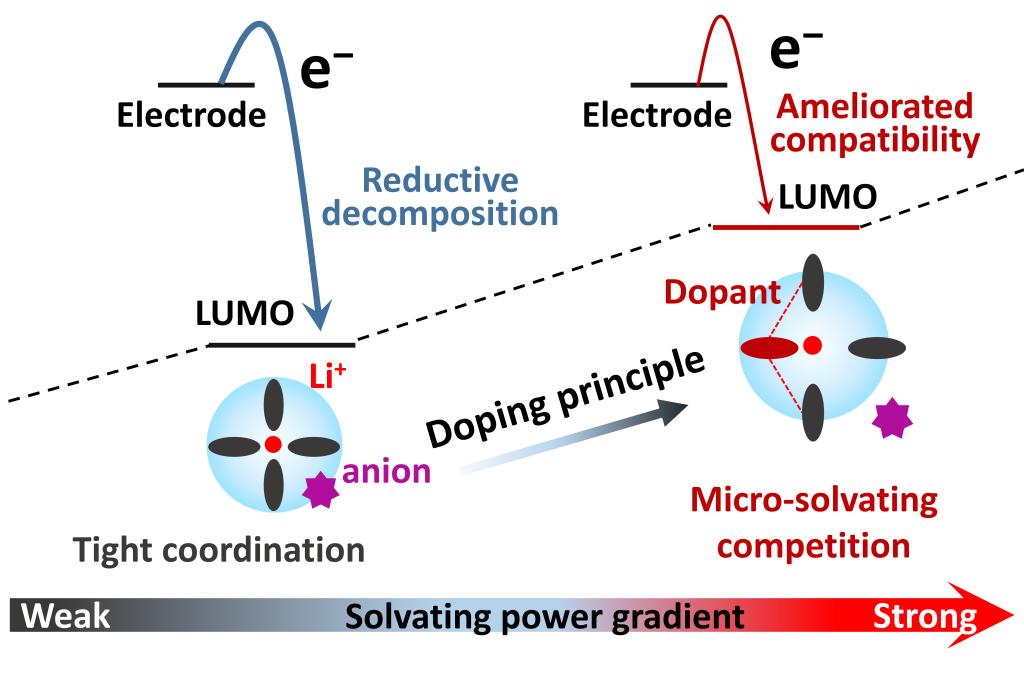
Operating Ni-rich cathode beyond 4.3 V safely holds promise for boosting energy density in lithium-ion batteries (LIBs). Methyl 2,2,2-trifluoroethyl carbonate (FEMC) shows oxidative stability and high safety but suffers from degraded LUMO energy levels once coordinated with Li+ within electrolytes. Here, we utilize propylene carbonate (PC) as a functional dopant, which deliberately tunes FEMC-dominated solvation chemistry and improves LUMO energy levels by dipole-dipole interaction and micro-solvating competition. As a result, the optimized electrolyte demonstrates expanded electrochemical window (4.7 V for NCM811), fire resistance and wide-liquid range (−60~120 ℃), affording 75.6% capacity retention in 1.2 Ah NCM811/graphite pouch cell over 1200 cycles. This “doping strategy” is generalized to other electrolytes (e.g., carbonates, fluorinated esters and carboxylic esters) and qualifies ameliorated interfacial compatibility, providing insights for designing high-safety electrolyte in high-energy LIBs.