《Research of diethyl ethylphosphonate-based flame-retardant wide temperature range electrolyte in lithium-ion batteries》
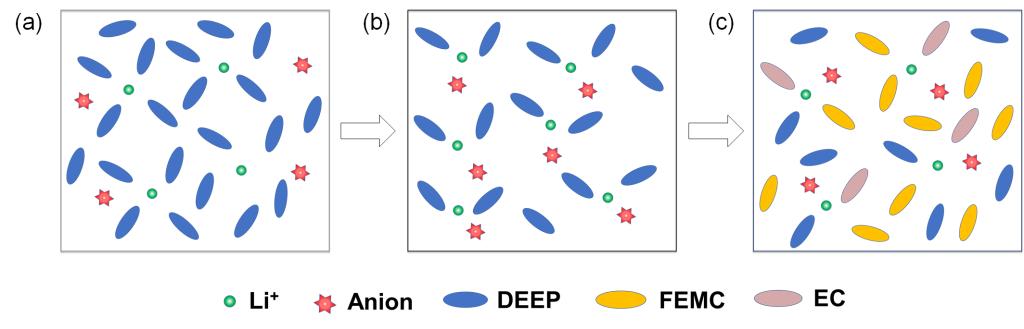
Lithium-ion batteries (LIBs) have been widely utilized in various applications, including electric vehicles and electrochemical energy storage. However, the safety concerns arising from the low flash point and flammability of commercial carbonate electrolytes hinder the widespread adoption of LIBs. Incorporating low-cost, non-flammable diethyl ethylphosphonate (DEEP) flame retardant into carbonate electrolytes effectively mitigates the risk of battery fires and explosions caused by electrolyte combustion. Nevertheless, the strong interaction between DEEP and Li+ often results in DEEP infiltrating the first solvated shell layer of Li+, thereby participating in the formation of the solid electrolyte interphase (SEI) on the graphite anode. Unfortunately, the SEI generated from the reductive decomposition of DEEP exhibits poor electron shielding capability, unable to prevent the continuous decomposition at the interface, consequently leading to graphite anode failure in DEEP-modified carbonate electrolytes. To overcome this obstacle, this study employs a synergistic approach utilizing ethylene carbonate as a strong ligand solvent and linear carbonate as weak ligand solvent. This combination aims to weaken the interaction strength between DEEP and Li+, thereby reducing the proportion of DEEP in the first solvated shell layer of Li+ and inhibiting DEEP decomposition on anode. In the formulated DEEP-modified carbonate electrolyte with conventional concentration (~1.15 mol L-1), the graphite anode exhibits a remarkable capacity retention of 95.6% after 150 cycles. Furthermore, the electrolyte maintains fluidity at -60 °C, while the Graphite/LiFePO4 battery shows a capacity retention of 49.3% after 50 cycles at -20 °C.