《Passivating Lithiated Graphite via Targeted Repair of SEI to Inhibit Exothermic Reactions in Early−Stage of Thermal Runaway for Safer Lithium-Ion Batteries》
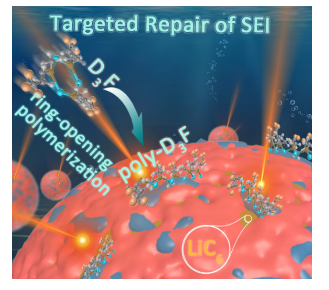
The exothermic side reaction between LiCx and electrolyte is not only the main cause of heat accumulation in the early stage of thermal runaway in lithium-ion batteries, but also the fuse that promotes the development of thermal runaway. Theoretically, the construction of SEI with high thermal stability can effectively inhibit the above reactions. However, SEI essentially contains various unstable organic components that undergo exothermic decomposition reactions at around 60°C, and there is currently no effective strategy to deal with early exothermic side reactions of thermal runaway. In this study, it was found for the first time that lithium graphite (LiCx) triggered ring-opening polymerization of 1,3,5−trimethyl−1,3,5−tris(3,3−trifluoropropyl)cyclotrisiloxane (D3F) above 70 °C, which could target LiCx in real time in the early stage of thermal runaway and efficiently inhibit its exothermic side reaction with electrolyte. As a result, a carbonate-based electrolyte that can target the repair of SEI was designed. The self-release heat temperature and thermal runaway trigger temperature of the pouch battery using this electrolyte increased from 159.6 °C and 194.2 °C to 300.5 °C and 329.7 °C, respectively. This work opens up a new way for the development of functional electrolytes that inhibit the exothermic side reactions of batteries and block thermal runaway reactions.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202217774
