《Low-potential Li2C2O4 prelithiation catalyzed by 2D MoN with dominant (002) crystal face for high-energy lithium-ion batteries》
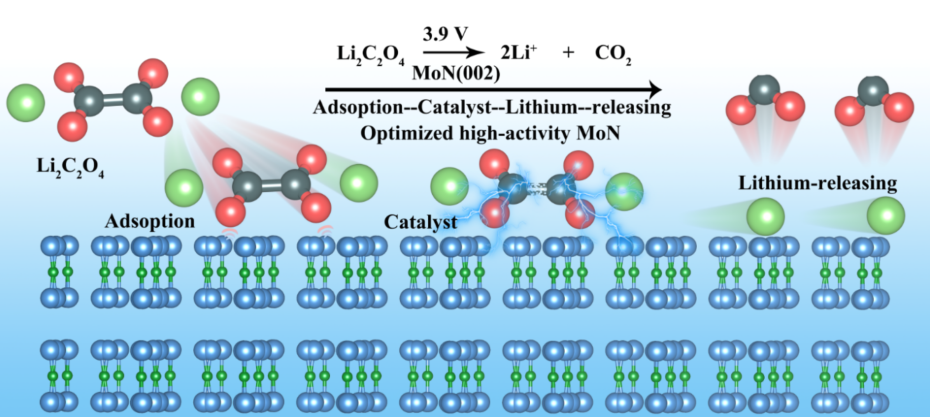
Lithium oxalate (Li2C2O4) is considered as an ideal prelithiation agent owing to its high specific capacity and cost-effectiveness. However, the high reaction energy barrier and low intrinsic conductivity lead to a lithium liberation potential higher than 4.5 V, which severely trials the tolerance of the electrolyte and electrodes and significantly compromises the battery performance. In this study, two-dimensional molybdenum nitride (MoN) with (002) dominant exposed crystal face having superior catalytic properties is engineered to promote the low-potential decomposition of Li2C2O4. With its half-filled electronic state of surface Mo atoms and ultra-high electronic conductivity, highly polarized MoN can effectively adsorb and catalyze the decomposition of Li2C2O4, thus reducing the lithium liberation reaction energy barrier by 46.8%. As a result, the lithium liberation potential of Li2C2O4 is diminished to 3.9 V. When paired with LiFePO4 (LFP) and LiNi0.5Co0.3Mn0.2O2 (NCM) cathode, forming Graphite || LFP-L and SiOx || NCM-L full cells, the capacity increase by 5.5 % and 11 %, respectively. Additionally, prelithiation facilitates the formation of a fluorine-rich solid electrolyte interphase (SEI) on the anode, markedly improving the cycling stability of the battery.