《Hunting highly conductive Li6PS5I electrolyte via Sn-Cl dual doping for solid-state batteries》
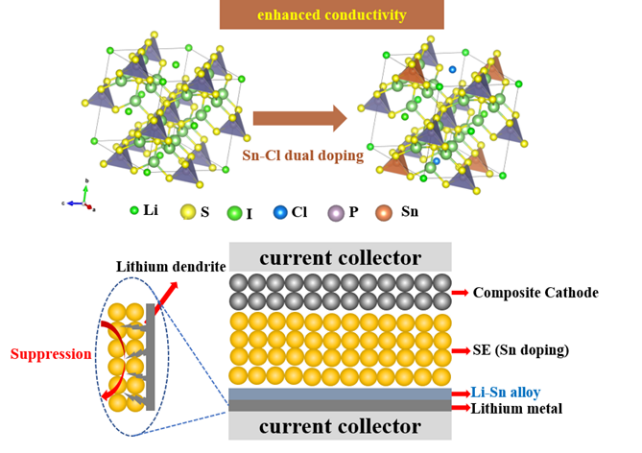
The low Li-ion conductivity and poor interfacial compatibility towards cathode/anode materials of Li6PS5I solid electrolytes have significantly impeded their applications in all-solid-state batteries. This work developed a facial design strategy to improve the ionic conductivity and lithium metal compatibility of Li6PS5I electrolyte by the Sn-Cl dual doping. By tailoring the dopant of Sn and Cl in the structure, the optimal Li6.3P0.9Sn0.1S5I0.8Cl0.2 electrolyte shows ultrahigh Li-ion conductivity up to 0.96 mS/cm at room temperature and enhanced lithium metal compatibility based on the lithium stripping/plating tests results. Due to the higher conductivity, the assembled all-solid-state battery using LiNi0.6Mn0.2Co0.2O2 cathode and Li-In anode delivers a high initial discharge capacity of 175.7 mAh/g at 0.1C and maintains 79.2% after 100 cycles. Moreover, the corresponding all-solid-state lithium metal battery using the Li6.3P0.9Sn0.1S5I0.8Cl0.2 electrolyte displays a higher capacity (137.7 mAh/g vs. 112.7 mAh/g) for the 1st cycle and superior cyclability performances than that using the pristine Li6PS5I electrolyte. EIS results confirm that the superior battery performances are attributed to the smaller interfacial resistances of those solid electrolyte-involved interfaces due to the Sn-Cl dual doping. This work demonstrates that the dual doping strategy is an effective route to explore new kinds of lithium argyrodite electrolytes with superior ionic conductivity and electrode compatibility.
https://www.sciencedirect.com/science/article/pii/S1359646222007138
