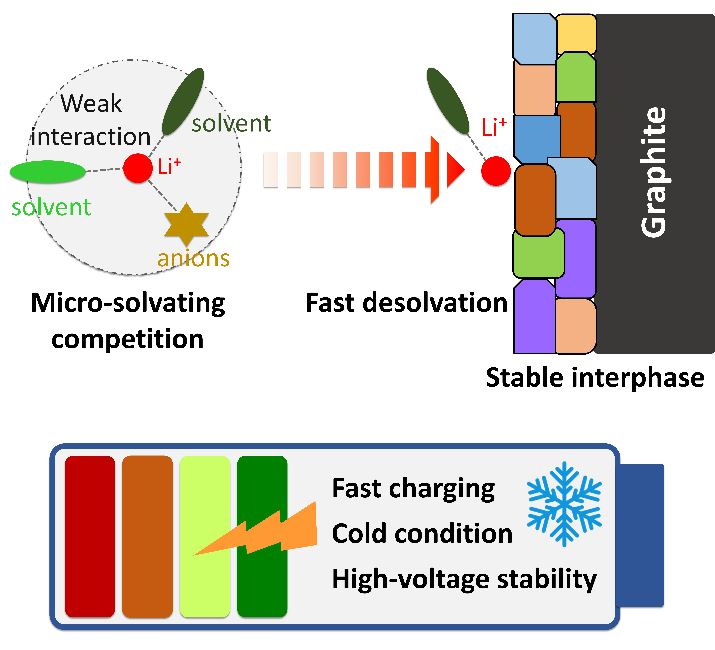
《Microsolvating competition in Li+ solvation structure affording PC-based electrolyte with fast kinetics for lithium-ion batteries》
Lithium-ion batteries (LIBs) suffer from energy loss and safety hazards under high-rate conditions, because of the sluggish electrochemical kinetics and unstable interfacial passivation. Herein, a PC-based electrolyte using weakly solvated solvent ethyl trifluoroacetate is developed to improve interfacial kinetics and stability in LIBs. A microsolvating competition is revealed in the bulk electrolyte, forming a loose Li+ coordination configuration with benign Li+ affinity and high ionic conductivity. Furthermore, an inorganic-rich interphase is constructed on graphite anode, affording smooth Li+ desolvation and reliable passivation. Consequently, the NCM622/graphite cell in PC-based electrolyte shows improved cycling stability (82.2% after 200 cycles) and rate capability (83% at 4C compared to 0.1C) at a high voltage of 4.5 V, much better than those of EC-based electrolyte (76.2% after 200 cycles and 74% at 4C). Additionally, the PC-based electrolyte affords reversible operation at –40 ℃ while the EC-based electrolyte fails at –40 ℃. This work highlights the potential of solvation structure engineering for low-energy-barrier electrolyte.