《Multilevel regulation of Li+-solvent interaction for fluorophosphate-based nonflammable electrolyte enabling lithium-ion batteries with long calendar life》
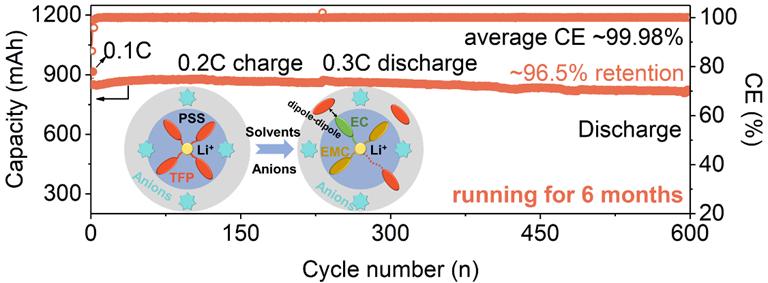
Carbonate-based electrolytes have been instrumental in extending the applicability of lithium-ion batteries (LIBs). However, their inherent high flammability contribute to frequent safety incidents, posing formidable challenges for the evolution of next-generation LIBs. Non-flammable electrolytes incorporating triethyl fluorophosphate (TFP) emerges as a promising solution. Nevertheless, TFP’s suboptimal reduction stability and limited electronic shielding of its reduction product impede compatibility with graphite (Gr) electrode. To address these challenges, we employ solvents as primary regulators to mitigate TFP-Li+ interaction. Simultaneously, anions serve as alternative regulators, preventing the formation of TFP-Li+ clusters during the cells’ dynamic operation. This multilevel regulation enhances TFP’s reduction stability throughout the entire lifespan of cells. Developed nonflammable electrolytes with high content of 83.3 vol.% TFP enable Gr anodes achieving approximately 92.7% capacity retention after 200 cycles. Practical 1Ah Gr||LiNi0.65Co0.15Mn0.2O2 pouch-cells deliver remarkable ~96.5% capacity retention after 600 cycles during 6 months of operation with a ~99.98% average CE. The extremely long-cycle calendar life and stability first verify the possibility of electrolytes containing high content TFP into commercial application. These findings not only introduce a fresh strategy for improving solvents’ reduction stability, but also contribute to the advancement of safer LIBs, marking a significant step forward in battery research.
https://www.sciencedirect.com/science/article/pii/S1385894724056353
