Congratulations to the doctoral student Qin Mingsheng for his paper entitled "Two-Dimensional Electrolyte Design: Broadening the Horizons of Functional Electrolytes in Lithium Batteries "was selected as a highly cited paper by ESI in 2024!
The increasing use of LIBs in modern society has necessitated the development of LIBs with superior performance including electrochemical reversibility, interfacial stability, efficient kinetics, environmental adaptability, and intrinsic safety, which is difficult to simultaneously achieve in commercialized electrolytes. Current electrolyte systems are essentially a solution containing Li salts (e.g., LiPF6) and solvents (e.g., ethylene carbonate and dimethyl carbonate), in which the latter dissolves Li salts and strongly interacts with Li+ (lithiophilic feature). Only lithiophilic agents can be functional modified (e.g., additives and solvents), which alters the bulk and interfacial behaviors of Li+ solvates. However, such approaches alter pristine Li+ solvation and electrochemical processes, making it difficult to strike a balance between electrochemical performance and other desired functions of electrolytes. Consequently, this common electrolyte design in lithiophilic solvents shows a strong coupling relation among formulation, coordination, electrochemistry and function of electrolytes. Fortunately, the invention of lithiophobic cosolvents (e.g., multifluorinated ether and fluoroaromatic hydrocarbons) has expanded the design space of electrolytes to lithiophilic (interacts with Li+) and lithiophobic (interacts with solvents but not with Li+) dimensions. Functional modifications switch to lithiophobic cosolvents, affording superior properties (carried by lithiophobic cosolvents) with little impact on primary Li+ solvation (dictated by lithiophilic solvents). This electrolyte engineering technique based on lithiophobic cosolvents is called the two-dimensional electrolyte (TDE) principle, which decouples formulation, coordination, electrochemistry, and function. The molecular-scale understandings of TDEs are expected to accelerate electrolyte innovations in the next-generation LIBs.
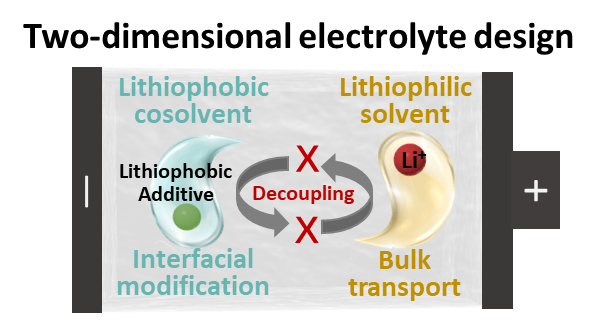

https://pubs.acs.org/doi/full/10.1021/acs.accounts.4c00022

 网站首页
>
正文
网站首页
>
正文